Research
Our group utilizes concepts and materials derived from both the natural and engineered worlds to create multi-functional platforms that seamlessly integrate with living bodies as next-gen sensors and actuators.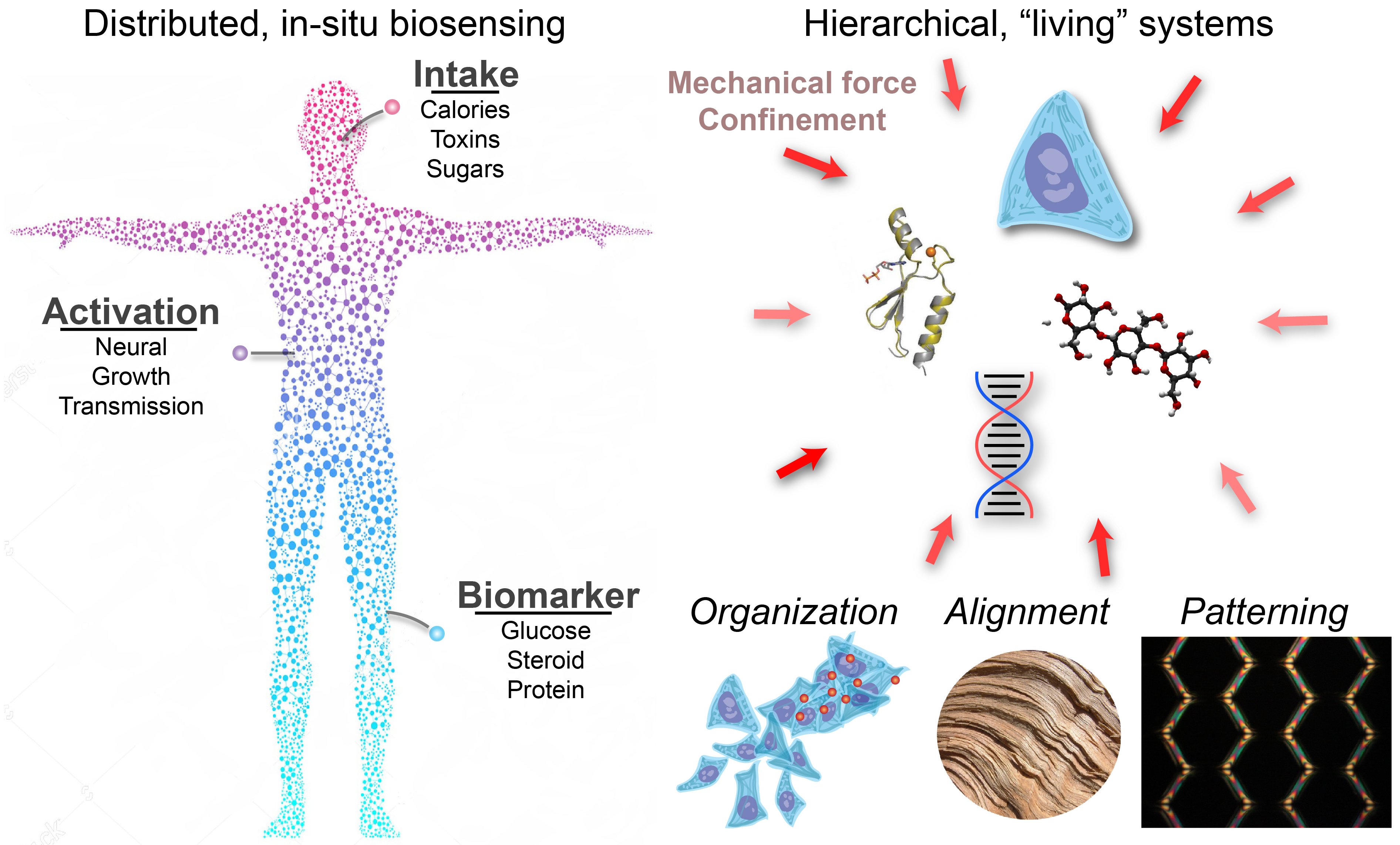
Wearables
Biomedical wearables are important because they enable continuous health monitoring and early detection of potential issues, empowering individuals to take proactive steps in managing their wellbeing. Additionally, these devices contribute to personalized healthcare, providing valuable data for healthcare professionals to tailor treatment plans and improve patient outcomes.
Bio-material
Bio materials are critical to research as they serve as the foundation for developing new medical treatments, drug delivery systems, and tissue engineering solutions, ultimately enhancing the quality of human life. Their unique properties and compatibility with living systems enable groundbreaking discoveries, paving the way for advancements in regenerative medicine, disease prevention, and overall healthcare innovation.
NANO-BIO
State-of-the-art nano-bio-electromagnetic interfaces have revolutionized the field of bioelectronics, enabling precise communication between biological systems and electronic devices at the nanoscale. These interfaces find applications in advanced diagnostics, targeted drug delivery, and neural prosthetics, offering innovative solutions to some of the most pressing healthcare challenges and improving the quality of life for millions of people.
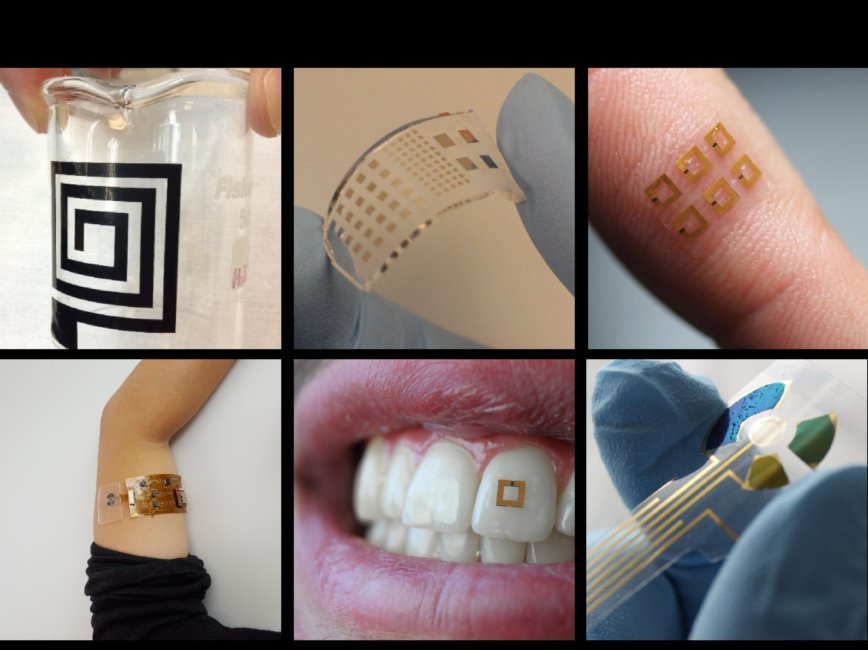
Biopolymer-enhanced, wearable devices
Our lab is integrating flexible and bio-responsive polymers into wearable devices to enhance biosensitivity, mechanical properties, and shelf-life.Recent advances in flexible wearable devices have focused on the development of low-form factor (or mobile format) systems to capture user data. These have culminated in all-inclusive devices that capture information from biofluids such as sweat or saliva. Despite technological growth in the integration of sensors with wireless or mobile platforms, the sensors at the core of such technologies have not significantly advanced and often possess major limitations that prevent their widespread adoption. This lab thrust employs a biomaterials-based strategy to evolve such wearable biosensors, potentially enabling long-term, body-mounted sensing of biometric data. Specific applications for these sensors include bio-sensitive RFid’s to monitor the properties of saliva in-situ, or stabilized enzymatic fuel cells to extract bioenergy from glucose. We plan to adapt our biosensors to readout new modes of biological behavior both in-vitro and in-vivo.
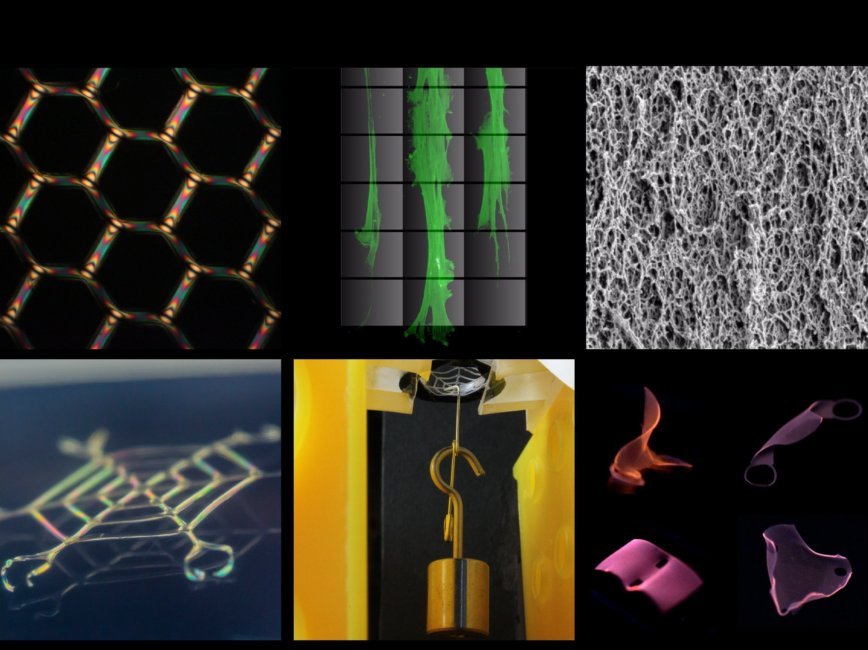
(bio) Materials-by-design
Our group co-opts the assembly strategies of “living” systems to build hierarchical, multi-material systems that possess programmability at the nano-, micro-, and macro-length scales.Biological constructs are prime examples of “materials by design,” whereby living structures are optimized for a variety of niches such as flight, fluid transport, or mechanical force transduction. Broadly, such constructs are formed via a simple mechanism: units (such as cells or polymers) are directed to assemble or grow under mechanical and chemical constraints. This mechanism is the fundamental inspiration behind mechanically-directed assembly, a technique realized by this lab that utilizes complex, pre-programmed mechanical strains on self-assembling (and shaped) biopolymers. This strategy results in porous, heterogeneous hierarchical constructs with structural control at the nano- to macro-scales. The realization of designer, porous microsystems is of fundamental importance to modern technology, as implementations of such devices bridge the gap between the solid, modular nature of engineered devices and the porous/textured, analog behavior of natural systems that gives rise to emergent biological function.
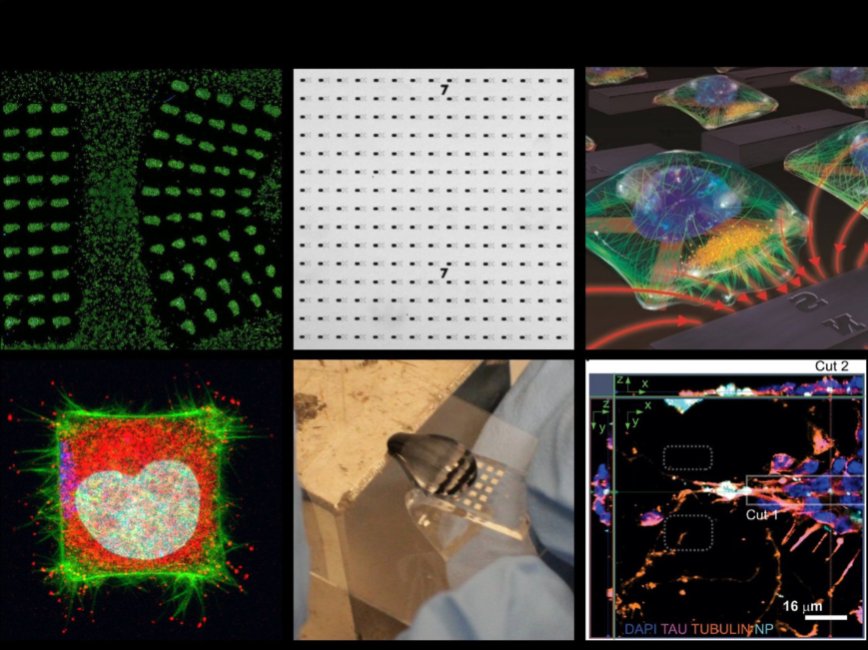
Nano-bio-electromagnetic interfaces
Our group builds nanoscale interfaces and control systems to manipulate the growth and development of living, cellular networks.In nature, organic life utilizes inorganic materials for chemical synthesis and in more specialized environments as sensors to detect aspects of their environment. One prime example of this is magnetotactic bacteria, which assemble Fe2O3 into worm-like constructs as an internal sensor to detect the direction of the Earth's magnetic field. The goal of this thrust is to engineer conceptually similar, hybrid (mixed organic-inorganic) nanoscale interfaces that can manipulate the growth of living systems non-invasively and remotely. One such approach developed by the lab controls cellular response by localizing signals from within the intracellular space. This is accomplished by using reconfigurable micro-electromagnetic fields to translate the positioning of cell-dosed magnetic nanoparticles. Early iterations of this technique have been used to generate large, programmable forces on cancer cells and neural networks. We plan to adopt recent optogenetic and magnetogenetic strategies in our evolving cell-control toolkit.

